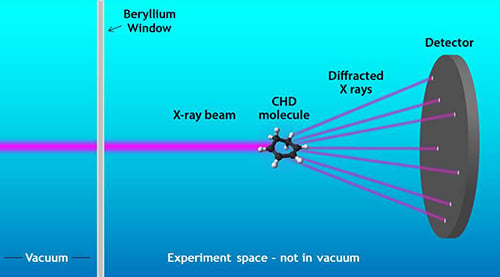
A beryllium window enables scientists to see chemical reactions never before possible.
Scientists at the U.S. Department of Energy's Stanford Lab Accelerator (SLAC) in California shared an exciting new application of beryllium. They have taken a "molecular movie" of a chemical reaction for the first time. The results of their research could give new insights into to how chemical bonds form, helping researchers better understand biological processes.
SLAC's molecular movie is enabled by a window made of our beryllium. The purity grades range from 98.5% to 99.8% and provide a number of properties that other materials can't match:
- Transmissive – The ability to let high energy X-rays pass through it unimpeded
- Tensile strength – The beryllium window must:
a) span an opening (aperture) while
b) withstanding different pressures on both sides (vacuum and non-vacuum)
- Temperature endurance – High energy x-ray beams could melt the window.
- Beryllium's high melting temperature (2,348° F) enables it to withstand SLAC's x-ray demands
Here's how it works: first, the chemical reaction is initiated by blasting the gas (1,3-cyclohexadiene) with a separate, high-powered optical laser. That breaks the molecular bonds, converting it into another gas called hexatriene. While the chemical transformation is still in action, the LCLS x-ray laser strikes the altered molecule, creating a distinctive diffraction pattern that's captured by a detector. The shape of the pattern on the detector helps scientists ‘infer back what's going on in the molecule'.
Once scientists can understand how to manipulate atoms, they can decide how to build stronger atomic structures for a wide variety of uses.